BLEOMYCIN INDUCED LUNG INJURY
21 AUG 2014
BLEOMYCIN INDUCED LUNG INJURY
Dr Lajeesh Jabbar, MEM PGY2, MIMS Calicut
Dr Shibu T Varghese, In-Charge, MIEM Calicut
Dr Venugopal PP, Director, Emergency Medicine, DM Health Care
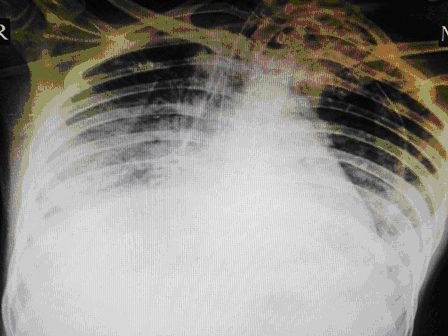
24 year old male, who is a K/C/O Embryonal Ca Testis- S/P Orchidectomy done 6 months back followed by chemotherapy with Bleomycin for 4 cycles, had presented with complains of cough since 1month and fever since 2 weeks. Associated breathing difficulty was also present and cough was productive. On arrival in the ED he was conscious ,oriented and talking .His vitals showed a HR of 125/min, BP of 90/60 and Saturation was 80%in Room Air. His RR was 35/min and was febrile. Work of breathing was greatly increased and bilateral coarse crepitations were present. Blood gases revealed Respiratory Acidosis. A trial of Non Invasive Ventillation (Bi PAP) was tried initially but failed. He was intubated immediately and put on pressure controlled ventilation.IV fluids were started and early antibiotics initiated. Initial CT Thorax showed features suggestive of Brochiolitis Obliterans organizing Pneumonia (BOOP).
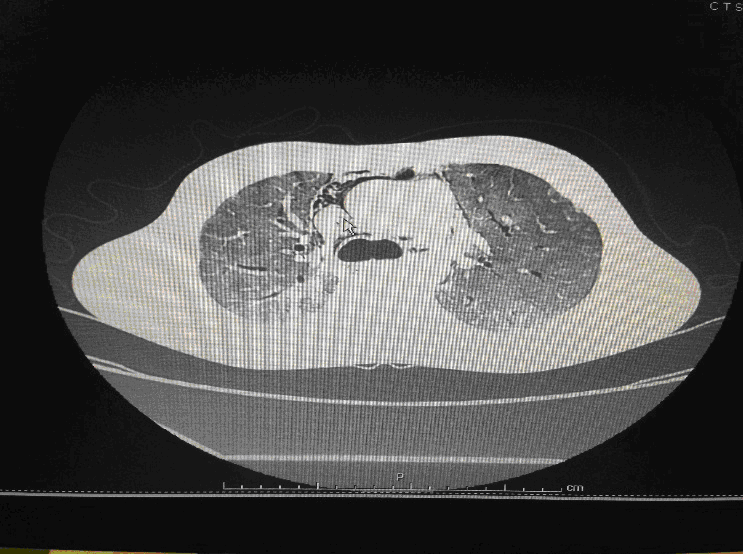
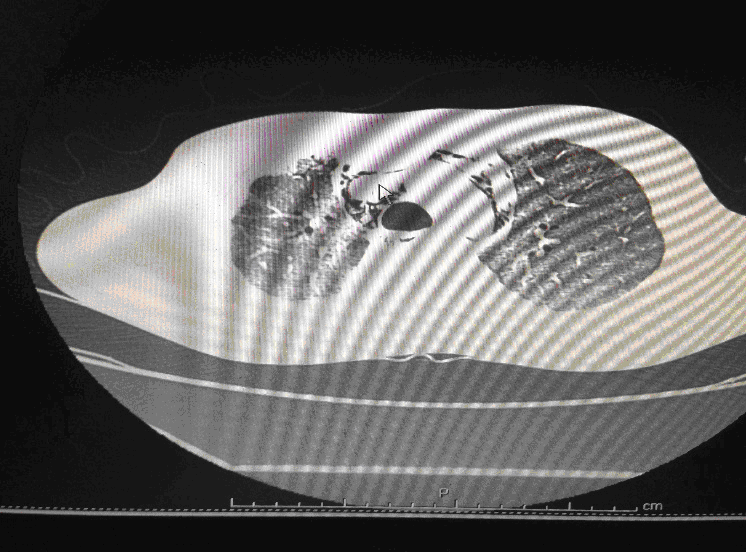
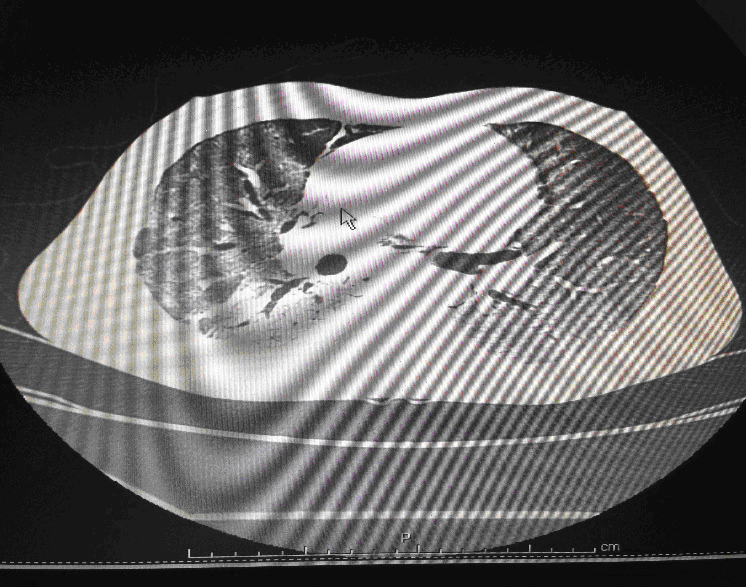
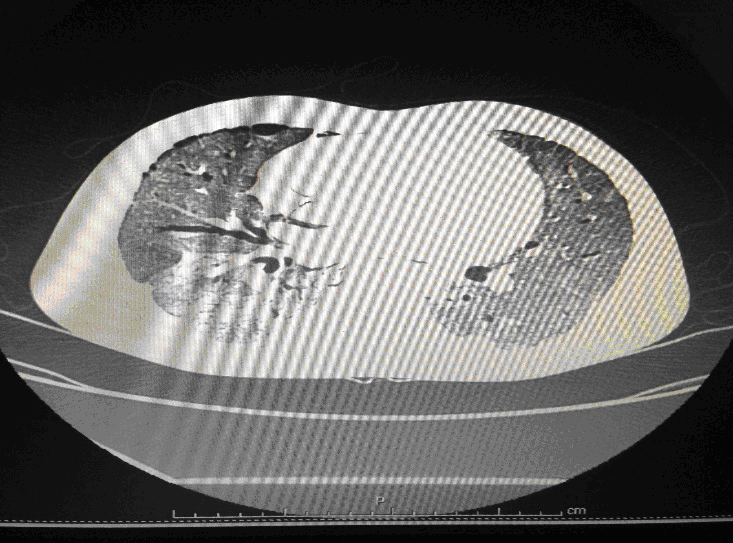
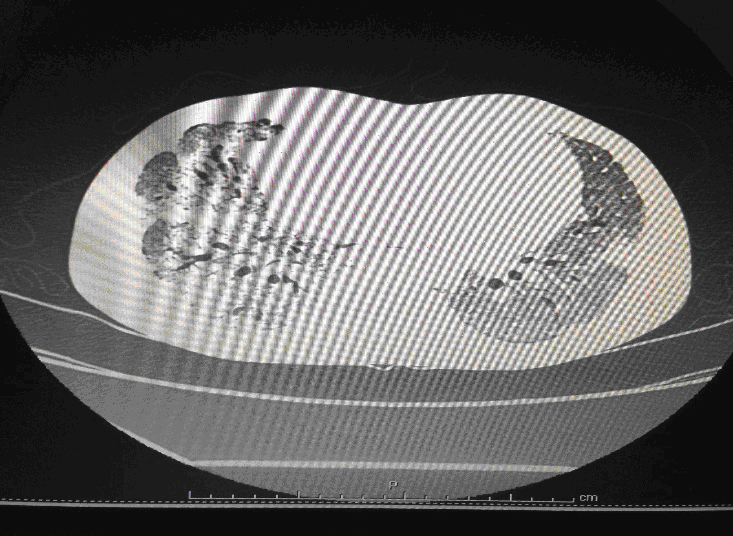
Following admission in the Medical ICU, Bronchoscopy was done and started on IV steroids and higher End Antibiotics. His bronchial wash grew ESBL Klebsiella and Pseudomonas. His other organ functions were not deranged. Repeat CT Thorax was taken which showed ground glass opacities, septal thickening, traction bronchiectasis, pleural thickening which was all S/o Drug induced Toxicity-Bleomycin.
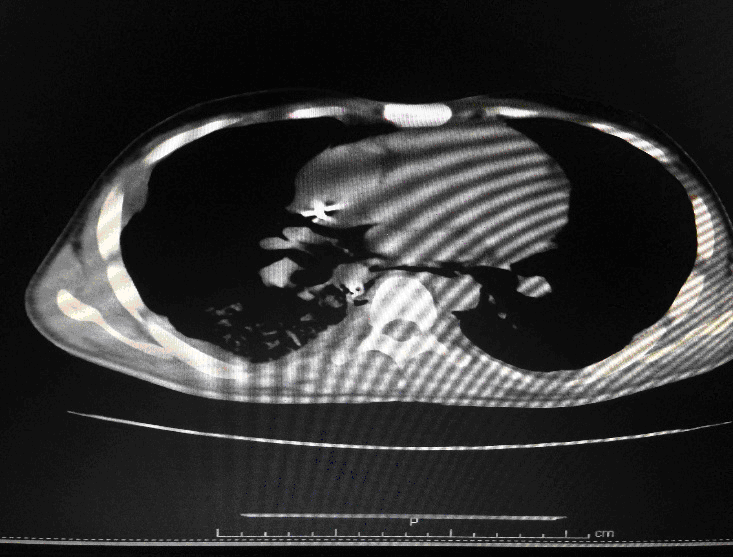
In view of the experimental reports of the efficacy of Imatinib in Bleomycin Induced Toxicity and Imatinib was started. However he continued to deteriorate with worsening oxygen parameters and high pressure requirements. He sustained cardiac arrest and could not be revived.
DISCUSSION:
Bleomycin is one of the first described chemotherapeutic agents and has been used for cancer treatment for many years. Despite the development of new drugs in oncology, bleomycin remains an important component of chemotherapy regimens for curable diseases such as germinative tumors and Hodgkin’s lymphoma. These neoplasias commonly affect young individuals, who may survive for long periods. In this regard, early diagnosis and treatment, and prevention of limiting toxicities such as bleomycin-induced lung injury, is crucial.
The major limitation of bleomycin therapy is usually pulmonary toxicity, which can be life threatening and has been described in up to 10% of patients receiving the drug. One of the potential determinants of bleomycin toxicity is bleomycin hydrolase, the enzyme that is primarily responsible for metabolizing bleomycin to nontoxic molecules. Interestingly, the two organs that are the most common sites of bleomycin toxicity (the lungs and the skin) have the lowest levels of the enzyme. Due to the feasibility of cloning the gene that encodes bleomycin hydrolase, studies are now needed to determine whether genetic variability in this enzyme accounts for individual susceptibility to or protection from bleomycin-induced pulmonary toxicity .
Several distinct pulmonary syndromes have been linked to the use of bleomycin, including bronchiolitis obliterans with organizing pneumonia (BOOP) , eosinophilic hypersensitivity, and, most commonly, interstitial pneumonitis, which may ultimately progress to fibrosis. The latter, bleomycin-induced pneumonitis (BIP), occurs in 0 to 46% of patients treated with bleomycin-containing chemotherapy, depending on the diagnostic criteria used . A reasonable estimate of BIP incidence is 10%. The mortality of patients with BIP has been reported to be approximately 10–20% in patients who develop bleomycin-induced lung injury (2-3% of all patients treated with bleomycin).
Typical chest radiographic findings are bilateral, bibasilar infiltrates, sometimes followed by diffuse interstitial and alveolar infiltrates. Fine nodular densities and subpleural opacification with volume loss and blunting of costophrenic angles may also be present. These early findings may evolve to progressive consolidation and honeycombing [23] Pneumothorax and pneumomediastinum are rare complications of bleomycin-induced pulmonary fibrosis .
High-resolution computed tomography (HRCT) of the chest is more sensitive than chest radiography in identifying lung abnormalities in bleomycin-exposed patients. HRCT patterns usually reflect the underlying histopathology . Diffuse alveolar damage is associated with airspace consolidation and ground-glass opacities. Findings suggestive of end-stage fibrosis include extensive reticular markings, traction bronchiectasis, and honeycombing. Organizing pneumonia manifests as ground-glass opacities in a bilateral but asymmetric pattern or by airspace consolidation with a subpleural or peribronchial distribution. Organizing pneumonia may occasionally present as one or more nodular densities that may mimic tumor metastases.
Bleomycin should be discontinued in all patients with documented or strongly suspected bleomycin-induced lung injury. Treatment with glucocorticoids is reserved for patients with symptomatic lung toxicity as spontaneous resolution of asymptomatic radiographic opacities has been described. Although no controlled studies in humans have systematically examined the efficacy of corticosteroids, a trial of these agents is probably warranted. Case reports and case series have described substantial recovery when significant inflammatory pneumonitis was present. The optimal dosing and duration of glucocorticoid therapy for bleomycin-induced lung injury are not known. Based on data from case series, most authors recommend initiating treatment with prednisone at 0.75 to 1 mg/kg. After four to eight weeks, the dose of prednisone is gradually tapered over an additional four to six months, in accordance with the patient’s condition and clinical response. Short-term improvement occurs in 50 to 70% of glucocorticoid-treated patients, but symptoms may relapse when therapy is tapered. Unlike the most common form of pulmonary pneumonitis, patients who present with disease patterns compatible with organizing pneumonia and hypersensitivity pneumonia are known to respond much better to corticosteroid therapy.
Sirolimus, an immunosuppressant used to prevent rejection of transplanted organs, was effective in reducing fibrosis score in a bleomycin-induced pulmonary fibrosis model, especially in the early phases of the disease.
A study by Wang et al. recently showed that gefitinib reduces pulmonary fibrosis induced by bleomycin in mice and suggested that administration of small-molecule epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors has the potential to prevent pulmonary fibrosis by inhibiting the proliferation of mesenchymal cells and that targeting tyrosine kinase receptors might be useful for the treatment of pulmonary fibrosis in humans.
Montelukast, a cysteinyl-leukotriene type 1 receptor antagonist used in the treatment of inflammatory lung disorders such as asthma, was studied in a mouse bleomycin-induced lung injury model. Treatment with montelukast significantly reduced the fibrotic area and hydroxyproline content in the fibrotic lungs of bleomycin-exposed mice. Montelukast exhibits its beneficial effects by inhibiting the overexpression of IL-6, IL-10, IL-13, and TGF-1.
Nilotinib has been approved for the treatment of chronic myeloid leukemia in patients with resistance or intolerance to imatinib. Like imatinib, nilotinib selectively inhibits the tyrosine kinase activity of PDGFR. In a therapeutic model, nilotinib showed more potent antifibrotic effects than imatinib.
References:
1. S. Sleijfer, “Bleomycin-induced pneumonitis,” Chest, vol. 120, no. 2, pp. 617–624, 2001.
2. J. A. D. Cooper Jr., D. A. White, and R. A. Matthay, “Drug-induced pulmonary disease. Part 1: cytotoxic drugs,” American Review of Respiratory Disease, vol. 133, no. 2, pp. 321–340.
3. Journal of Cancer Research - Volume 2013 (2013), Article ID 480608